Abstract
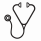
Thrombopoietin receptor agonists (TRA) have shown efficacy in clinical trials for patients with immune thrombocytopenia (ITP), achieving initial response rates of up to 88%. However, there is limited research on the effectiveness of TRA in routine clinical practice. The primary objective of this retrospective, observational study was to describe the proportion of patients who achieve platelet counts [plts] ≥30,000/µl within 12 weeks of TRA initiation (treatment response; R). Secondary objectives of this study were to assess the safety and tolerability of TRA, the duration of treatment, maintenance of R, whether patients are able to come off treatment (TRA discontinuation) and, whether different treatment responses are observed in primary versus secondary ITP.
Patients aged ≥ 18 years with ITP (primary or secondary) who had initiated TRA (eltrombopag [EPG] or romiplostim [RPM]) treatment ≥4 months prior to data collection were included in the study from UK secondary and tertiary care centres. Data on patient characteristics, disease history, TRA treatment pathways, response to treatment and adverse events (AEs) were collected from medical records (observation period from date of ITP diagnosis to data collection).
Overall 267 eligible patients from 15 centres were included (primary ITP n=218, secondary ITP n=49); 42% (n=112/267) and 58% (n=155/267) of patients received EPG and RPM respectively as the first prescribed TRA. The study sample comprised 52% females (n=139/267); median (interquartile range [IQR]) age at TRA initiation was 56 years (40-70). The median (IQR) time from diagnosis of primary and secondary ITP to first TRA treatment was 4.0 years (0.9-11.7) and 1.9 years (0.2-9.5), respectively. 26% (n=57/ 218) of patients with primary ITP and 46% (n=22/48, no diagnosis date recorded for n=1) of patients with secondary ITP received their first TRA within 1 year of diagnosis.
Proportion of patients achieving and maintaining R, discontinuing treatment and median time to achieve first R for all patients are described in the table below. 23% (n=61/267) of all patients received both TRAs during the observation period, with 10% (n=6/61, n=5 initiated with RPM) patients switching to receive both TRAs at the same time. 23% (n=36/155) of all patients initiated on RPM switched to EPG and 22% (n=25/112) of all patients initiated on EPG switched to RPM.
AEs (related to the use of TRA) were recorded in 33% (n=31/95) and 23% (n=28/123) of patients with primary ITP receiving EPG and RPM respectively. 12% (n=31/267) of all patients discontinued their initial TRA due to poor response (n=20), adverse events (n=7) or complications (n=4). Of the remaining 236 patients, 20% (n=48/236) patients discontinued their initial TRA due to physician/patient request (n=31) or for an unrecorded reason (n=17). 8% (n=14/267) patients discontinued their initial TRA due to reasons other than those listed above and data was unavailable for 2% (n=6/267) patients.
17% (n=36/218) of patients with primary ITP discontinued TRAs within one year of initiation (15% [n=14/95] on EPG and 18% [n=22/123] RPM). 39% (n=19/49) of patients with secondary ITP discontinued TRA within one year of treatment initiation (47% [n=8/17] on EPG and 34% [n=11/32] RPM). 67% (n=147/218) of patients with primary ITP and 43% (n=21/49) of patients with secondary ITP were still receiving a TRA at the time of data collection.
Of the 237 patients achieving R, 21% (n=49/236) discontinued TRA treatment within one year of initiation. The median (IQR) time to discontinuation of TRA after achieving R for all patients, was 34 (13 - 97) weeks.
The results from this retrospective observational study suggest that patients with primary and secondary ITP respond to TRA in real world settings in a manner that is consistent with results from clinical trials. These results also show that greater proportions of patients with secondary ITP are able to discontinue TRA treatment within one year of initiation suggesting the benefit of these treatments in ITP patient care.
Cooper:Amgen, Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Provan:Amgen, Novartis: Honoraria, Research Funding. Scully:Novartis: Honoraria, Other: Member of Advisory Board, Speakers Bureau. Grech:Novartis: Other: sponsorship for scientific meetings. Bagot:Amgen, Novartis: Speakers Bureau. Hill:Novartis: Honoraria; Novartis: Honoraria. Nokes:Bayer, MSD, Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Ramscar:Novartis: Employment. Saunders:pH associates: Employment.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal